Introduction
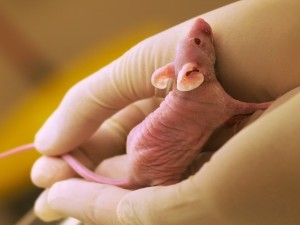
Researchers who thanked Cancer Research UK (CRUK) for their ‘generous financial support’ conducted cruel and outdated experiments on a number of nude mice, in order to give them bone cancer.(1) The animals, some 6 weeks old and others 16 weeks old, had cancer cells injected into their hearts. The male mice received prostate cancer cells and the female mice received breast cancer cells. These cells were made to glow so that tumour growth could be identified while the animals were alive. (This was also confirmed after their deaths.) The animals were killed at various times after the injection into their hearts; the method of killing is not stated.
Animal Suffering
- The animals subjected to these procedures were ‘BALB/c nude mice’. These mice are described in a paper about the health of ‘experimental laboratory mice’(2) as having health problems such as aggression in males, heart problems and eye problems – including where the edges of the eyelids become inflamed and where abscesses form around the eyes. These poor animals also suffer from age-related hearing loss.
- The researchers injected cancer cells directly into the mice’s hearts. They do not state whether the animals were anaesthetised for this procedure. According to a paper by researchers in the US,(3) animals should be anaesthetised prior to the injection and animals should also be kept in pathogen-free conditions, but this is not mentioned in the paper.
- There is no description of how the animals were kept, whether they were caged alone, had bedding or enrichment, what they were fed or how much light they had each day. The US paper states that, after the injections into their hearts, animals should be kept under a warming lamp or on a heating pad and monitored continuously until they have completely recovered. The paper does not state whether or not this happened.
The experiments
- Three weeks after the cancer cells were injected, 80% of the young male mice had bone tumours as did 20% of the older animals. Five weeks later, 40% of the older animals were affected.
- Three weeks after the cancer cells were injected, 80% of the young female mice had bone tumours as did 50% of the older animals. The researchers state the young female mice had a ‘burden’ of tumours in their bones that was about 56 times higher than that of older mice.
- The authors report how some animals suffered tumours around or inside the heart due to ‘incorrect injections’.
- The authors cite evidence from human patients: ‘More importantly, these data correlate with disease outcomes in cancer patients: Breast cancer patients presenting at a younger age (<40) have worse survival rates and more metastases compared to disease presented in older women, while younger men (<44) with high grade prostate cancer, have poorer prognosis compared to older men with a similar grade/stage distribution’. This shows that comparable data is available from people so it is not clear why animals were being used.
- The weakness of animal ‘models’, which has been pointed out many times by Animal Aid, is acknowledged by the authors; ‘The xenograft model used in this study has limitations’. They go on to explain that, as the animals had no thymus, ‘we could not determine the role of the immune system’ in regulating the bone cancer spread. This is a very important element to exclude as this is the body’s way of fighting disease.
- Animal Aid has frequently explained how animal ‘models’ of disease bear little, if any, resemblance to how humans contract disease. The authors admit that their method of creating cancer in these animals is very different to the human situation: ‘the administration of a single high dose of cancer cells via intracardiac injection only partially mirrors seeding events in humans where low numbers of cancer cells are released into the circulation over prolonged time intervals.’ In these experiments, the males were injected with approximately 100,000 cancer cells and the females with approximately 75,000 cells in one injection. Again, huge differences in the animal ‘version’ of a human disease have not stopped researchers using animals.
Scientific critique
- The authors state in their first line that animal and human studies suggest that the frequency of cancer spreading to the bones is higher in those with a higher bone turnover, although they say the reason for this is ‘unclear’. The authors conclude that their studies suggest higher frequencies of bone cancer ‘are linked to increased bone turnover’, which is exactly what they state at the beginning has been indicated by previous studies.
- The animals used in this research were described by one source as having ‘a defective immune system because of a genetic mutation.’ And that they ‘are often used in cancer research because they do not reject tumor cells, from mice or other species.’(4) Most human patients will not have a defective immune system, so will be able, to some degree, to fight the cancer cells, unlike the poor animals used in these experiments.
- In the background information, the authors state that ‘Elevated levels of bone turnover have been shown to be correlate with increased numbers of metastases, in various xenograft models and clinical studies’. This shows that bone turnover has not only been investigated in other animals in laboratories, but also in humans, making this work pointless.
- The researchers state that ‘Cohorts of animals a minimum of 6’ were killed at the different time points, but there is no explanation of what this means, whether there may be more animals, and if so, how many and why. We believe that omitting such information is sloppy and unscientific.
- A paper from 2011 explains how a person’s genetic background could ‘increase or decrease the likelihood that a tumor will metastasize’.(5) Again, this is another difference between humans and animals in laboratories, especially animals such as those used in this research who are inbred and have a defective immune system.
- A paper from 2013, two years before this paper was published, states that certain nutritional deficiencies in mice affect the bone and skeletal tumour growth. They state that accelerated bone turnover, is ‘a dominant factor in promoting breast and prostate cancer growth in bone’(6) Again, it would appear that other authors had already reached the conclusion of the link between bone turnover and the spread of cancer.
Additional information and background notes
- The aim of the experiment was to determine whether the spread of cancer to bone was due to the number of cancer cells arriving at specific sites in the body or due to more cancer cells being caused to divide by the environment of the bone.
- Metastatic bone cancer is cancer which has spread to the bone from other parts of the body.
- Cancer Research UK (CRUK), according to their annual report and accounts for 2015/2016, had an income of £635 million, with legacies accounting for £178 million of this.(7)
- To find out about Animal Aid’s campaign, which calls on the public to leave legacies only to humane charities and highlights the importance of legacies to charities such as CRUK, visit the Don’t leave a legacy of suffering campaign page
References
- Wang, N. et al (2015) ‘The frequency of osteolytic bone metastasis is determined by conditions of the soil, not the number of seeds; evidence from in vivo models of breast and prostate cancer’, Journal of Experimental and Clinical Cancer Research, vol.34, pp.124 (DOI 10.1186/s13046-015-0240-8)
- Burkholder, T et al (2012), ‘Health evaluation of Experimental Laboratory Mice’, Current Protocols in Mouse Biology. Vol.2, pp.145-165.
- Park, S.I. et al (2010) ‘Pre-Clinical Mouse Models of Human Prostate Cancer and their Utility in Drug Discovery’, Current Protocols in Pharmacology, doi:10.1002/0471141755.ph1415s51.
- https://www.cancer.gov/publications/dictionaries/cancer-terms?cdrid=44579
- Mathot, L. & Stenninger, J. (2011) ‘Behavior of seeds and soil in the mechanism of metastasis: A deeper understanding’, Cancer Science, vol.103, pp.626-631.
- Zheng, Y. et al (2013) ‘The role of the bone microenvironment in skeletal metastasis’, Journal of Bone Oncology, vol.2, issue.1, pp.47-57
- http://www.cancerresearchuk.org/sites/default/files/cruk_annual_report_and_accounts_201516.pdf
